- 6 June 2022
- Posted by: nemcatgroup
- Category: Publications
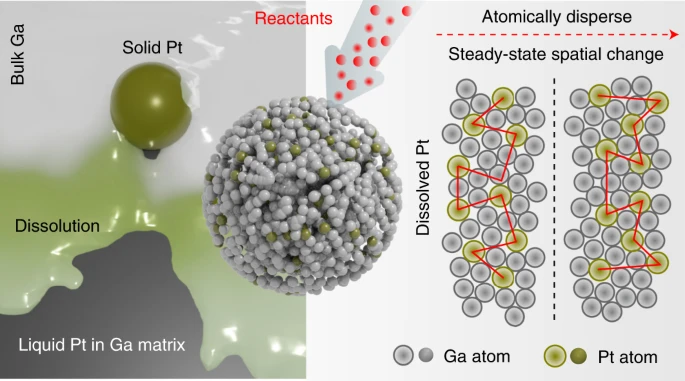
Insights into metal–matrix interactions in atomically dispersed catalytic systems are necessary to exploit the true catalytic activity of isolated metal atoms. Distinct from catalytic atoms spatially separated but immobile in a solid matrix, here we demonstrate that a trace amount of platinum naturally dissolved in liquid gallium can drive a range of catalytic reactions with enhanced kinetics at low temperature (318 to 343 K). Molecular simulations provide evidence that the platinum atoms remain in a liquid state in the gallium matrix without atomic segregation and activate the surrounding gallium atoms for catalysis. When used for electrochemical methanol oxidation, the surface platinum atoms in the gallium–platinum system exhibit an activity of ∼2.8×107 mAmgₚₜ⁻¹, three orders of magnitude higher than existing solid platinum catalysts. Such a liquid catalyst system, with a dynamic interface, sets a foundation for future exploration of high-throughput catalysis.
