- 19 March 2019
- Posted by: nemcatgroup
- Category: Publications
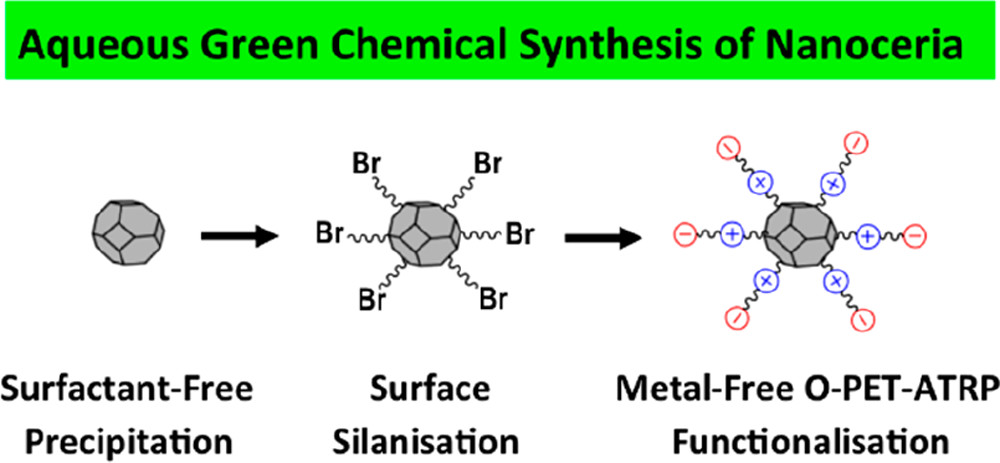
The present work reports a new method for the green chemical synthesis of biomaterials using an integrated, room-temperature, aqueous, chemical technique involving surfactant-free precipitation of nanoceria, surface silanization, and functionalization with zwitterionic agents by metal-free organocatalyzed photoinduced electron transfer atom-transfer radical polymerization (O-PET-ATRP). The synthesis mechanism for each of these steps is presented. The present work is the first to report the use of water, rather than organic solvent, as medium for O-PET-ATRP. The nanoparticles were characterized by FTIR, laser Raman microspectroscopy, XPS, TGA, TEM, and NMR. The functionalization resulted in retention of nanoparticle shape, hindrance of plasma protein adsorption, maintenance of small hydrodynamic size, and establishment of a near-electroneutral surface. The latter demonstrates that the zwitterion formed a continuous passivating layer on the nanoceria surfaces. These outcomes resulted in higher uptake of functionalized nanoceria and enhanced redox performance. Nanoceria provided cytoprotection to normal cells while cytotoxicity was observed in fibrosarcoma cells. Nanoparticles generated pH-controlled redox responses in fibrosarcoma cells where, at physiological conditions of pH 7.4, antioxidant activities were observed while prooxidant behavior was generated at tumor microenvironment conditions of pH 6.4. These effects were accentuated at both pH values for functionalized nanoceria, which is a direct result of the functionalization.
