- 27 February 2017
- Posted by: nemcatgroup
- Category: Publications
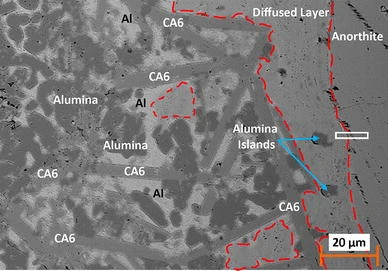
The kinetics of the anorthite–Al system have been examined by exposing anorthite to pure Al at 850–1150 °C for 0.5–250 h. The interfaces were investigated by electron microscopy (SEM, EDS, EPMA, and TEM). The results showed that Si4+–Al3+ interdiffusion and associated oxygen vacancies plus Ca2+–Al3+ interdiffusion and associated calcium vacancies drove the anorthite → CA2 and the CA2 → A (alumina) transformations, respectively, at 850 and 950 °C. At 1050 and 1150 °C, increased solubilities of silicon and oxygen in the liquid Al resulted in significant formation of CA2, which, when in contact with anorthite, leads to formation of gehlenite. Si4+–Al3+ interdiffusion was identified as the controlling process of the anorthite–Al interactions and so it has been quantified in terms of the activation energy of Q = 112 kJ/mol and the diffusion coefficient pre-factor of D 0 = 4 × 10−8 m2/s.
