- 19 May 2015
- Posted by: nemcatgroup
- Category: Publications
No Comments
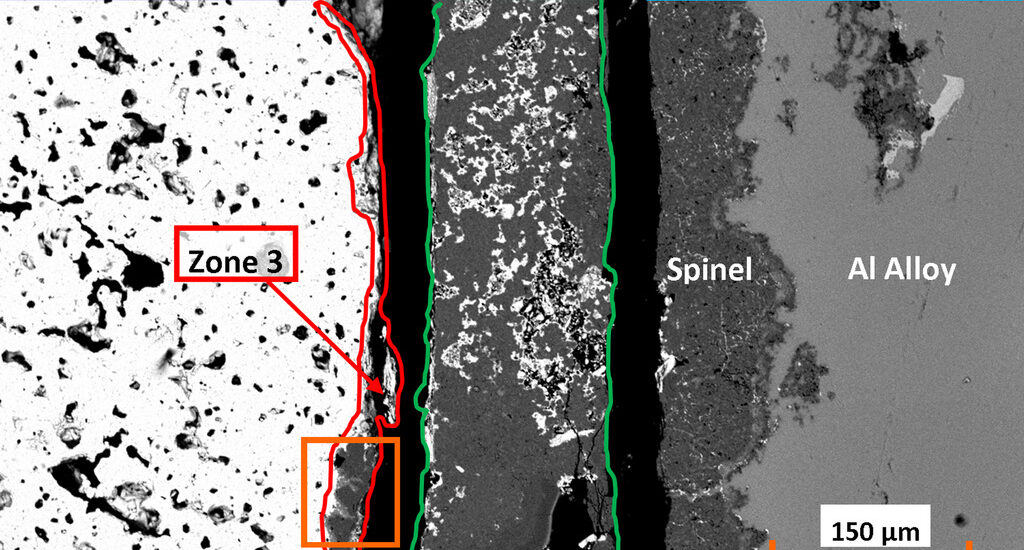
A corrosion cup test was undertaken using BaAl2Si2O8 and Al4.1Zn3.2Mg alloy, heated in air for 150 h at 850°C. Electron probe microanalysis, X-ray diffraction, and scanning electron microscopy coupled with energy dispersive spectroscopy were used to identify the mineralogical and microstructural changes at the interfaces. The microstructural results revealed three microstructural areas: (1) Spinel layer with large numbers of Al alloy channels; (2) interfacial area with mainly alumina, spinel, and BaAl2Si2O8; and (3) interdiffusion zone chemically close to barium hexaaluminate. The principal observations are:
-
- BaAl2Si2O8 was highly resistant to molten Al alloy corrosion owing to sluggish kinetics, as evidenced by the observation of unreacted BaAl2Si2O8 grains in the interfacial area.
- The nature of the microstructure, particularly an interdiffusion zone instead of a continuous layer of precipitated alumina at the interface between the Al alloy channels and the unreacted BaAl2Si2O8 supports the conclusion that the corrosion mechanism is governed by interdiffusion (Si/Ba and Al/Mg) and substitution.
- The formation and limited retention of an MgO layer at the metal-ceramic interface played a critical role in alloy oxidation and the consequent interfacial phenomena.
