- 2 January 2015
- Posted by: nemcatgroup
- Category: Publications
No Comments
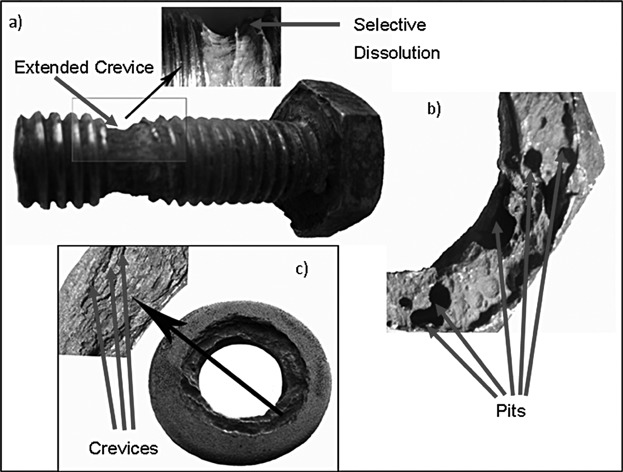
The corrosion of duplex stainless steel 2205 was investigated both under service conditions and via an electrochemical study in chlorinated media. The steel sample surfaces showed pitting, crevice and selective dissolution, with the ferrite phase being more susceptible to dissolution in this media. The dissolution of MnS was identified to be a contributing factor for pitting corrosion, while electrochemical investigations revealed that the presence of hypochlorite ions caused a switching from a general corrosion phenomenon to a localized one.
